Background
Acute myeloid leukemia (AML) is a clonal aggressive hematologic neoplasm characterized by arrested myeloid maturation, resulting in the accumulation of myeloid blasts in bone marrow, blood, and other tissues. Cytogenetic and molecular characteristics further stratify AML into favorable, intermediate, or high-risk classifications; with high-risk including DEK-NUP214, KMT2A-rearrangement, BCR-ABL1, GATA2, MECOM, deletion 5q, abnormal 17p, complex or monosomal karyotype, wild type NPM1 and FLT3-ITD, or mutated: RUNX1, ASXL1, or TP53. In fit and eligible patients, therapy for high-risk AML patients involves induction therapy, and response evaluation at count recovery. If remission is confirmed, it is ideal that the patient proceed straight to hematopoietic stem cell transplantation (HSCT). HSCT remains the only curative therapy currently available. The logistics of proceeding to transplant might necessitate consolidation chemotherapy usually involving high dose cytarabine (HiDAC).
Methods
We performed a retrospective cohort analysis and evaluated outcomes for high-risk AML patients treated at our transplant institution, the Georgia Cancer Center, who were diagnosed and received HiDAC consolidation therapy between November 2003 and December 2020. High-risk AML was defined as those patients, whose AML was positive for one (or more) of the following FLT3, monosomy 5,7 and/or complex cytogenetics (CG) defined as having greater than or equal to 3 CG changes). For those not transplanted, we reviewed the complications, if any, that would preclude a HSCT.
Results
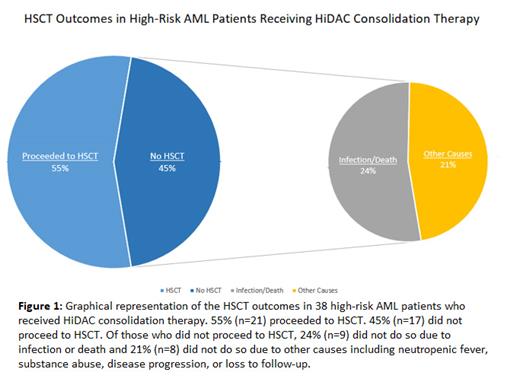
A total of 93 patients diagnosed with high-risk AML were evaluated at our center from 2003 to 2020. Males comprised 46.2% (n=43) and females comprised 53.8% (n=50) of the cohort. Of these patients, the median age of the patient cohort was 61.5 years. 40.8% (n=38) received HiDAC consolidation. Among patients who received HiDAC consolidation and proceeded to undergo HSCT, the median age was 47.6 years. In contrast, patients who received HiDAC consolidation but were unable to undergo HSCT had a median age of 61.3 years. Among the patients who were unable to undergo HSCT due to death, the median age was 41.8 years. For patients who received HiDAC consolidation but did not undergo HSCT due to reasons other than death, the median age was 53.4 years. 55.3% (n=21) went on to HSCT, and others did not proceed to HSCT. Of the patients who did not receive HSCT, 76.4% (n=13), underwent HLA-typing, indicating consideration for transplantation. Of these 17 patients (44.7%, n=9) did not proceed to transplant due to infectious complications, the remaining 8 had noninfectious reasons for non-transplant. Of the 9 patients without HSCT due to infection, four had an infection that prevented them from receiving a transplant and five patients passed away prior to transplant due to septic shock or multi-organ failure. Infections included bacteremia from Klebsiella, E. Coli, Proteus, group B strep, vancomycin resistant E. Faecalis, and coagulase negative staph, pneumonia from Klebsiella, urinary tract infections from extended spectrum beta-lactamase resistant E. Coli, Klebsiella, and proteus mirabilis, fungal sinusitis, and Clostridium difficile colitis. Other reasons that prevented HSCT included neutropenic fever with an unknown cause, substance abuse, disease progression, and lack of follow-up.
Conclusion
The data from our study and the literature on this topic over the past 30 years suggest starting the process of HSCT during the induction admission. HSCT is the sole curative option for high-risk AML patients, and the concept of bridging the period between induction and HSCT with HiDAC consolidation appears to offer no additional benefit compared to IDAC. Moreover, this approach prevents a subset of this population from being fit enough to receive HSCT.
Disclosures
Keruakous:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees. Cortes:Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Biopath Holdings: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Research Funding; Forma Therapuetic: Consultancy; Gilead: Consultancy; Takeda: Consultancy, Honoraria. Kota:Novartis: Honoraria; Kite: Honoraria; Pfizer: Honoraria; Incyte: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal